CBIS LM Core: InVi SPIM Lattice Pro
Bruker Luxendo
invi spim lattice pro
The InVi SPIM Lattice Pro light sheet microscope provides a high level of flexibility for illuminating samples while maintaining ease of use and system stability. The beam shape can be tailored to your specific sample requirements – large field of view, high speed or optimal spatial resolution.
Location: CBIS Light Microscopy Core (S1A #01-04 CBIS lab)
Booked CBIS equipment before? Information for first-time users
About InVi SPIM Lattice Pro
Light sheet microscopy is well-suited to live imaging of relatively fast biological processes due to its relatively high speed, high signal-to-noise ratio, low phototoxicity and low photobleaching. The lattice light-sheet microscope takes the modality one step further, into the realm of super-resolution.
(Above) wild-type (left) and diseased (right) heart of zebrafish in high-speed video. Samples prepared by Cathleen Teh (lab of Thorsten Wohland); imaging by Cathleen Teh and Lin Yangchen.
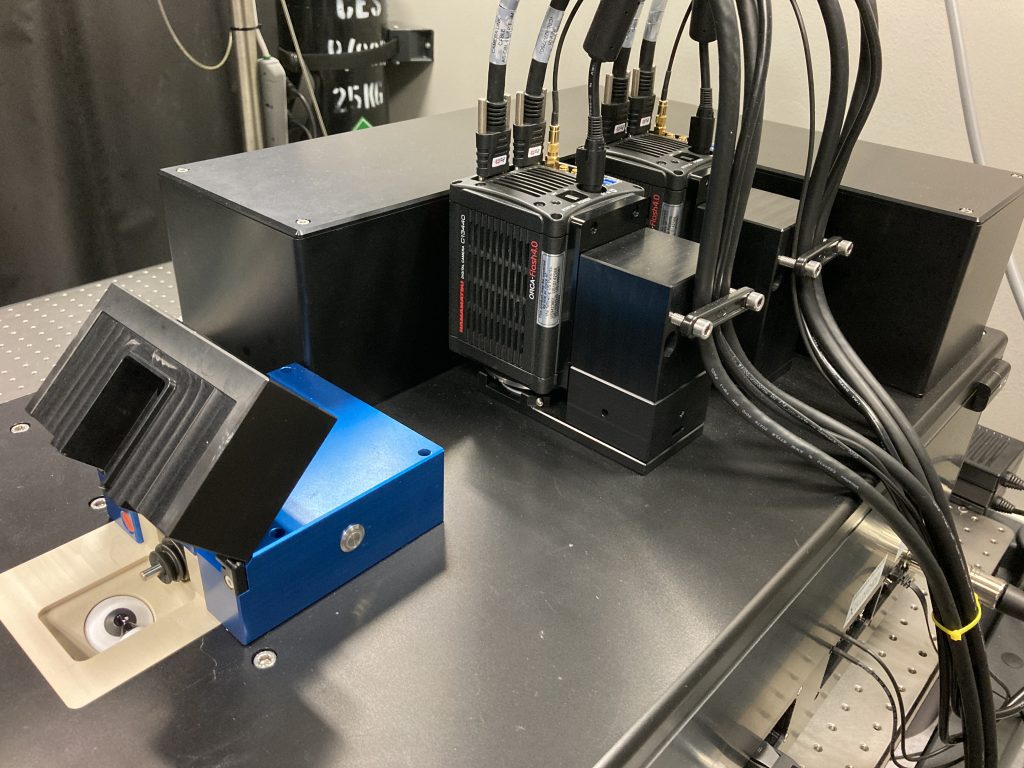
The InVi SPIM Lattice Pro has one illumination objective and one detection objective. It can accommodate both thin and relatively thick samples thanks to the long working distance (approx. 2 mm) of the detection objective. The inverted configuration allows the use of recessed holders that position samples deeper into the space between the objectives than is possible with regular slides or dishes. This allows the use of an objective of high NA.
(Above) maximum-intensity Z-projection of dynamics of U2OS cells. Sample prepared by Zhu Chennianci (lab of Liou Yih-Cherng); imaging and deconvolution by Zheng Chao and Goh Wei Jia.
Beam patterns are generated by a spatial light modulator that has been calibrated to correct for wavefront aberrations. The measured FWHM of sub-resolution beads imaged with the thinnest Gaussian beam are 288 by 320 by 672 nm. Lattice patterns make super-resolution SIM possible. Users can also install their own SLM patterns.

The system is also capable of imaging fluorescence correlation spectroscopy at up to about 10,000 frames a second (small ROI) using the Fiji ImFCS plugin developed by our Biophysical Fluorescence Laboratory.
- CBIS quick start guide
- CBIS comprehensive user manual (28 May 2025)
- Manufacturer’s guide
- Bacterial biofilm preparation (by Rutuparna Kulkarni)
Features
- Illumination objective: Special Optics 28.6× NA 0.7 water dipping, chromatic correction 440-660 nm
- Detection objective: Nikon CFI Apo 25× W (water dipping) NA 1.1 with motorized refractive index correction collar
- Magnifications (via motorized tube lenses): 31.3× (FOV 420 µm), 62.5× (FOV 210 µm)
- Diode lasers (nm): 405, 488, 515, 561 (diode-pumped solid-state), 642
- Laser safety class 1
- Beams: Gaussian, Bessel, droplet, optical lattices, user-defined
- Lattice-based structured illumination microscopy (SIM)
- SLM pattern format: 800 × 600-pixel 8/16-bit greyscale PNG
- Two Hamamatsu ORCA-Flash4.0v3 sCMOS (technical documentation)
- Emission filters (camera short):
- BP 418–462
- LP498
- BP 500–530
- BP 526–564
- LP572
- BP580–627
- BP655–704
- empty8
- empty9
- empty10
- Emission filters (camera long):
- LP 498
- BP 500–530
- BP 526–564
- LP 572
- BP 580–627
- LP 656
- BP 655–704
- empty8
- empty9
- empty10
- Dichroic mirrors:
- LP 499
- LP 560
- empty 1
- Sample incubation: temperature, humidity, carbon dioxide
Applications
- Embryogenesis
- Organoids
- Neurobiology
- Cell culture
- Plant science
- High-throughput FCS
Software
- LuxControl
- Micro-Manager
- Tokupic
- SIMToolbox
- SSPIM toolbox
- arivis Vision4D
- Fiji
- Huygens
