CBIS LM Core: 3I diSPIM
3i diSPIM
Marianas LightSheet
Live imaging directly involves inevitable tradeoffs of spatial resolution, temporal resolution and phototoxicity. This is especially true when interrogating imaging in three dimensions and/or three-dimensional samples over time (3D and/or 4D imaging) to obtain a complete picture of many dynamic subcellular processes. Selective Plane Illumination Microscopy (or Light Sheet Microscopy) uses a separate excitation lens perpendicular to the widefield detection lens to illuminate only the plane of interest within sheet of light. Its low photo toxicity, photo bleaching, high signal-to-noise ratio and high imaging speed make it a favourite tool for live imaging.
Location: CBIS Light Microscopy Core (S1A #01-04 CBIS lab)
Booked CBIS equipment before? Information for First Time Users
About Marianas LightSheet
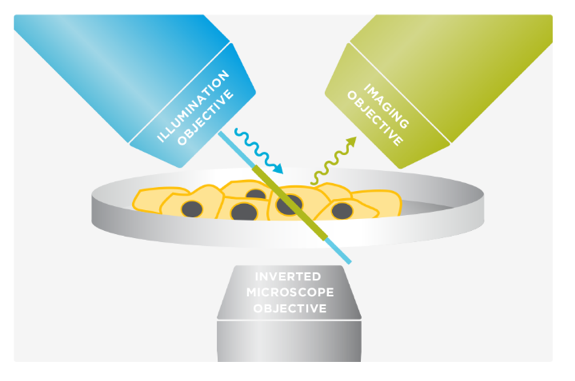
Marianas LightSheet™ from Intelligent Imaging Innovations, Inc. (3I, USA) merges dual inverted selective plane illumination (diSPIM) with the power and flexibility of a live-cell microscopy system. It employs two orthogonal objectives positioned at 45° above the specimen plane. By alternating between the objectives for imaging and excitation, diSPIM captures two volumes that are fused and deconvolved to achieve isotropic resolution. Unlike capillary-based light sheet methods, diSPIM allows for standard specimen preparation in standard dishes and standard media.
The 3I diSPIM at CBIS/DBS confocal core, offers a single- (iSPIM) or dual- (diSPIM) sided light-sheet arrangement on an inverted microscope with custom built OKO environment chamber for live cell imaging and photon manipulation unit for FRAP. 3D isotropic resolution of 360 nm in x-y-z is achievable (by beads). Samples are prepared on conventional glass coverslips and agarose gel for monolayer cell culture and small organisms imaging respectively.
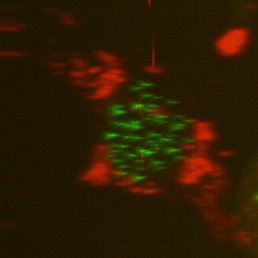
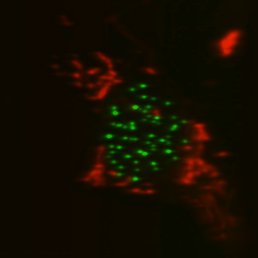
Top, single-sided light sheet; bottom, diSPIM with isotropic resolution plus deconvolution. U2OS cell with CENP-A and tubulin. Sample: Zemin Jiang, Assoc Prof Yih-Cherng Liou.
Help
- Quick Reference
- Manual
- Live zebrafish sample preparation protocol (Dr Cathleen Teh)
- Live cell sample preparation and mounting protocol
- Multiview reconstruction (Slidebook)
- Multiview reconstruction (Fiji)
- Deconvolution (Huygens)
Features
- Modalities: Single and dual inverted SPIM (iSPIM and diSPIM)
- iSPIM x-y-z resolution: 360 x 360 x 760 nm
- diSPIM x-y-z resolution: 360 x 360 x 360 nm
- SPIM objectives: Nikon water dipping objectives: CFI NIR Apo 40X/0.8 NA WD 3.5 mm, x2; CFI Plan Fluor 10x/0.3NA WD 3.5mm, x2.
- Finder objectives: Zeiss 40x and 10x
- Sample platform: Rectangular coverslips (1.5#, 24 x 50 mm) or agarose on petri dishes
- Excitation sources: 405nm (100mW), 488nm (50mW), 561nm (50mW) and 640 nm (100mW)
- Dichroic mirror: Shemrock410/504/582/669 nm BrightLine® quad-edge dichroic beamsplitter
- Emission filters Shemrock440/521/607/700 nm BrightLine® quad-band bandpass filter
- Light-sheet camera: Twin Hamamatsu ORCA-Flash4.0 V3 sCMOS
- Environmental control: diSPIM custom designed OKO incubator with temperature, CO2 and humidity control
- Software: Slidebook 6
- Operating system: Windows 10
- Data transfer: 10 GB network connection – no external hard drives allowed
- Usage: Staff-assisted and self-use allowed (following training)
Applications
- 3D-5D Live cell imaging: multi-channel, 3D and time lapse imaging for isotropic resolution
- 3D-5D Live small organism imaging
- Advanced F-techniques: FRAP
Software
- 3i Slidebook