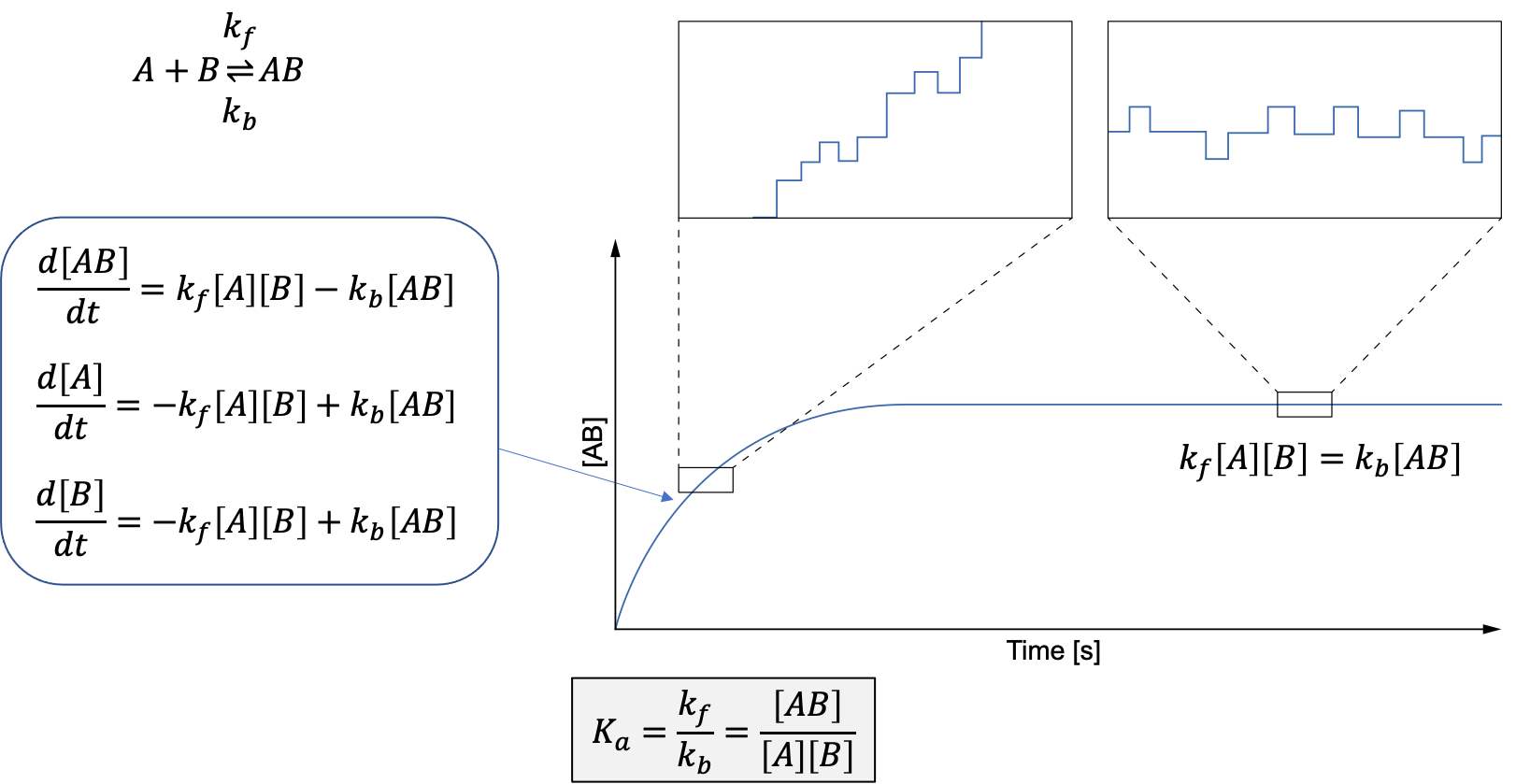
In a chemical system there are constant fluctuations, be it thermal ones or ones driven by chemical reactions. In the case of chemical reactions, fluctuations in concentrations of educts and prodcuts are created by forward and backwrd reactions. At the start of a reaction the forward reactions will dominate. Once equilibrium is reached, there are as many forward as backward reactions. The fluctuations in both cases, though, are the same, and are determined by the forward and backward reaction rate constants. It is easier to measure kinetics in the non-equilibrium state as the changes are laregr and unidirectional. But if we have single molecule sensitivity, we can measure at equilibrium and extract the same information as from kinetcis. FCS is exploiting this fact and is measuring fluctuations in a system in equilibrium with single moelcule sensitivity. It then extracts the information about molecular processes from the fluctuations.

Although FCS was originally designed to look at chemical reactions it is most commonly used nowadays to measure molecular mobilities. For that purpose, we create a small observation volume in which fluorescent molecules can be excited and detected. When fluorescent molecules move through the observation volume they create fluoresence fluctuations which FCS can analyze to extract molecular mobilities and concentrations. The observation volume needs to be small so that at the concentration of interest (in Biology typically in the range of nM - μM) not too many molecules are present so that the signal from single molecules entering and leaving the volume can be detected).