
CHRISTOPH WINKLER
Associate Professor
Contact Information:
Department of Biological Sciences
National University of Singapore
14 Science Drive 4
Block S1A, Level 6
Singapore 117543
Lab webpage: Winkler’s Lab
6516 7376
6779 2486
dbswcw@nus.edu.sg
Research Areas
Developmental Biology, Neurogenetics, Molecular Cell Biology
Research Interests
- Using zebrafish models to study disease mechanisms of Spinal Muscular Atrophy (SMA)
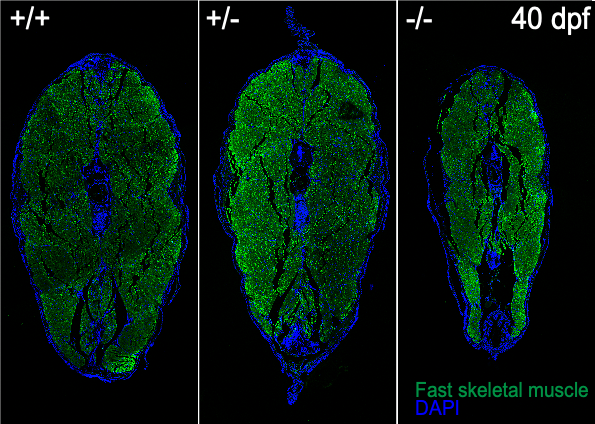
SMA is a neurodegenerative disease and is one of the leading genetic causes for infant mortality. It is caused by low levels of a ubiquitous protein, SMN, and severely affects the neuromuscular system with a characteristic motor neuron degeneration. We generated a zebrafish mutant line zfsmnA6T that recapitulates human intermediate Type II SMA using CRISPR-Cas9. This line demonstrates a progressive loss of SMN protein (to ~20%) at late juvenile and early adult stages.
The zfsmnA6T mutants have demonstrated that there might be a defect in the maintenance of the pre-synapse at later developmental stages. Hence, we are interested in identifying essential candidate genes that might be involved in the maintenance of pre-synaptic nerve endings, Schwann cell dynamics and motoneurons development.
Utilizing the optical clarity of zebrafish embryos, we can capture various developmental processes in real time through non-invasive imaging techniques to identify the role of potential SMN downstream targets in motoneurons development, synaptogenesis etc. We can also examine the formation and the maintenance of neuromuscular junctions and axonal transport in SMN-deficient motor neurons in zfsmnA6T mutants.
- Understanding the mechanism for axonal transport in developing neurons

Axonal transport is a process where motor proteins would actively delivery cargoes like organelles along the microtubules, from one end of the axon to the other, and this process is essential for neuronal development and survival. Disruptions in the transport of cargos have been reported in a range of neurodegenerative disorders and we wanted to understand the molecular aspects on how axonal transport is important on maintaining the integrity of neurons as the organism develops.
Together with the use of the zebrafish animal model, we have several genetic and molecular tools to enable us on visualising and understanding how specific proteins could be integral for the axonal transport mechanism.
- In vivo characterization of zebrafish Mdk/Ptn-Ptprz1 signalling in embryogenesis

Midkine (MDK) and its paralog pleiotrophin (PTN) are secreted heparin-binding growth factors. They are believed to function in several biological processes, such as embryogenesis and metastasis, through binding to receptor protein tyrosine phosphatase zeta 1 (PTPRZ1). Due to an additional whole-genome duplication, zebrafish possesses two mdk, mdka and mdkb, one ptn, and two ptprz1 genes, ptprz1a and ptprz1b, which implies potential sub- and neo-functionalization with different ligand-receptor combinations.
In this project, we utilize CRISPR-Cas9 system to generate mdk/ptn knockout zebrafish mutants as loss-of-function models and mdk/ptn knockin lines to visualize endogenous Mdk/Ptn dynamics in vivo.
These models allow us to gain further understanding on the dynamics of Mdk/Ptn diffusion and their binding to Ptprz1 in vivo under the help of cutting-edge live imaging and biochemical techniques. The availability of such observation provides new insight in explaining the functions of Mdk/Ptn-Ptprz1 signalling during zebrafish embryogenesis in vivo.
- In vivo imaging of osteoblast-osteoclast interaction in a medaka model for osteoporosis
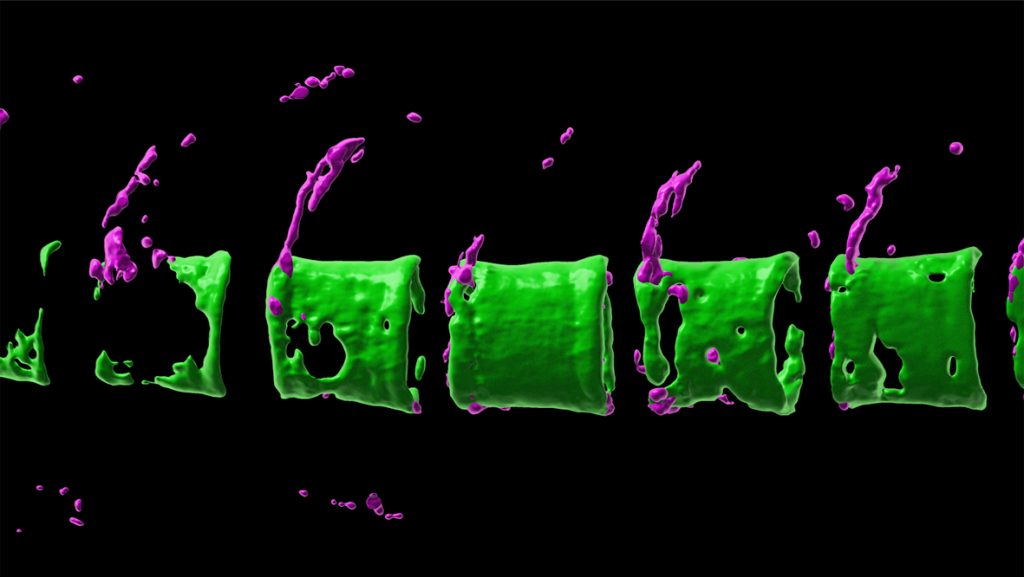
We are interested in the cellular mechanisms that control bone homeostasis. Fish, such as medaka, have bone cells very similar to humans. Also, the genetic networks regulating formation of bone-forming osteoblasts and bone-resorbing osteoclasts are highly conserved.
We have established several transgenic medaka lines that express fluorescent reporters in bone cells at distinct stages of differentiation, or express RANKL, an osteoclast-inducing factor, under control of a heatshock promoter. Upon heatshock, RANKL induces the formation and activation of ectopic osteoclasts. This results in degradation of bone matrix in a manner very similar to the situation in human osteoporosis patients.
This unique in vivo model allows visualization of osteoblast/osteoclast interaction in an intact living animal during bone degradation as well as regeneration.
- A medaka model for Schmid Metaphyseal Chondrodysplasia (SMCD)
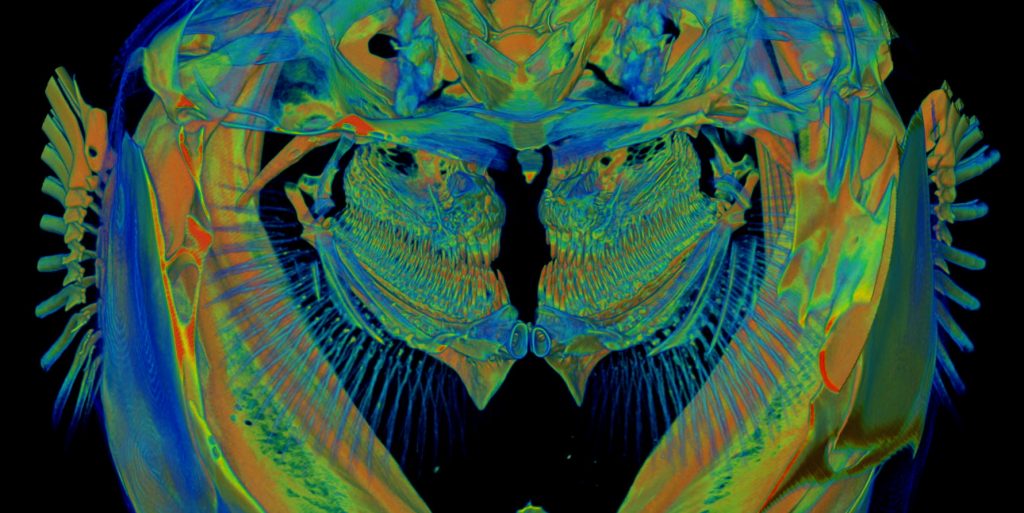
SMCD is a skeletal disease associated with growth plate abnormalities, hip deformities and dwarfism. At present, the pathomechanism of SMCD remains unclear and there is no cure for this disease. To elucidate molecular mechanisms underlying SMCD, we generated a medaka mutant which phenocopies SMCD.
Using high-resolution imaging techniques such as live confocal microscopy, X-ray tomographic microscopy and electron microscopy, we found that cell polarity is impaired in the SMCD medaka mutant. Further investigation into the cause and consequences of disrupted cell polarity in the medaka mutant may shed light on the pathogenesis of SMCD and facilitate development of future SMCD therapies.
Selected Publications 2019-2024
-
Kuhl, H., Tan, W.H., Klopp, C., Kleiner, W., Koyun, B., Ciorpac, M., Feron, R., Knytl, M., Kloas, W., Schartl, M., Winkler, C.*, Stöck, M.* (2024). A candidate sex determination locus in amphibians which evolved by structural variation between X- and Y-chromosomes. Nature Comms 15(1):4781. *co-corresponding authors.
-
Tan, W.H., Ruecklin, M., Larionova, D., Tran, B.N., van Heuven, B.J., Marone, F., Matsudaira, P., Winkler, C. (2024). A Collagen10a1 mutation disrupts cell polarity in a medaka model for Metaphyseal Chondrodysplasia type Schmid. iScience 27(4):109405.
-
Tan, W.H., Winkler, C. (2024). Lineage Tracing of Bone Cells in the Regenerating Fin and During Repair of Bone Lesions. Methods Mol Biol 2707:99-110.
-
Tzung, K.W., Lalonde, R.L., Prummel, K.D., Mahabaleshwar, H., Moran, H.R., Stundl, J., Cass, A.N., Le, Y., Lea, R., Dorey, K., Tomecka, M.J., Zhang, C., Brombacher, E.C., White, W.T., Roehl, H.H., Tulenko, F.J., Winkler, C., Currie, P.D., Amaya, E., Davis, M.C., Bronner, M.E., Mosimann, C., Carney, T.J. (2023). A median fin derived from the lateral plate mesoderm and the origin of paired fins. Nature 618(7965):543-549.
-
Trumpp, M., Tan, W.H., Burdzinski, W., Basler, Y., Jatzlau, J.*, Knaus, P.*, Winkler, C.* (2023). Characterization of Acvr1/Acvr2 Activin receptors in medaka (Oryzias latipes): Towards establishing a novel animal model for Fibrodysplasia Ossificans Progessiva. PLoS One 18(9):e0291379. * co-corresponding authors
-
Imangali, N., Sokolova, V., Kostka, K., Epple M., Winkler, C. (2023). Functionalized calcium phosphate nanoparticles to direct osteoprotegerin to bone lesion sites in a medaka (Oryzias latipes) osteoporosis model. Frontiers Endocrinology 14:1101758.
-
Mo, J., Wan, M.T., Au, D.W., Shi, J., Tam, N., Qin, X., Cheung, N.K.M., Lai, K.P., Winkler, C., Kong, R.Y., Seemann, F. (2023). Transgenerational bone toxicity in F3 medaka (Oryzias latipes) induced by ancestral benzo[a]pyrene exposure: Cellular and transcriptomic insights. J Environ Sci 127, 336-348.
-
Phan, Q.T., Chua, Y.K., Jin, A., Winkler, C.*, Koh, W.P.* (2022). CXCL9 predicts the risk of osteoporotic hip fracture in a prospective cohort of Chinese men – a matched case-control study. Journal Bone Mineral Research 37, 1843-1849. * co-corresponding authors
-
Tan, W.H., Winkler, C. (2022). A novel non-disruptive and efficient knock-in allows fate tracing of resident osteoblast progenitors during repair of vertebral lesions in medaka. Development 149(12):dev200238.
-
Liu, R., Imangali, N., Ethiraj, L.P, Carney, T.J., Winkler, C. (2022). Transcriptome profiling of osteoblasts in a medaka (Oryzias latipes) osteoporosis model identifies Mmp13b as crucial for osteoclast activation. Frontiers in Cell and Developmental Biology 10:775512.
-
Mo, J., Au, D.W.T, Guo, J., Winkler, C., Kong, R.Y.C., Seemann, F. (2022). Benzo[a]pyrene osteotoxicity and the regulatory roles of genetic and epigenetic factors: A review. Critical Reviews in Environmental Science and Technology 52 (18), 3244-3282.
-
Ethiraj, L.P., Fong E.L.S., Liu, R., Winkler, C., Carney, T.J. (2022). Colorimetric and fluorescent TRAP assays for visualising and quantifying fish osteoclast activity. Europ Journal of Histochemistry 66, 3369ff.
-
Tay, S.H., Ellieyana, E.N., Le, Y., Sarusie, M.V., Grimm, C., Ohmer, J., Mathuru, A., Fischer, U., Winkler, C. (2021). A novel zebrafish model for intermediate type spinal muscular atrophy demonstrates importance of Smn for maintenance of mature motor neurons. Hum Mol Genet 30(24):2488-2502.
-
Koh, A., Sarusie, M.V., Ohmer, J., Fischer, U., Winkler, C.#, Wohland, T.# (2021). Fluorescence Correlation Spectroscopy Reveals Survival Motor Neuron Oligomerization but no Active Transport in Motor Axons of a Zebrafish Model for Spinal Muscular Atrophy. Frontiers in Cell and Developmental Biology 9:639904.
-
Foo, Y.Y., Motakis, E., Tiang, Z., Shen, S., Lai, J.K.H., Chan, W.X., Wiputra, H., Chen, N., Chen, C.K., Winkler, C., Foo, R.S.Y., Yap, C.H. (2021). Effects of Extended Pharmacological Disruption of Zebrafish Embryonic Heart Biomechanical Environment on Cardiac Function, Morphology and Gene Expression. Dis Model Mech 250(12):1759-1777.
-
Fraher, D., Mann, R.J., Dubuisson, M.J., Ellis, M.K., Yu, T., Walder, K., Ward, A., Winkler, C., Gibert, Y. (2021). The endocannabinoid system and retinoic acid signaling combine to influence bone growth. Mol Cell Endocrinology 529:111267.
-
Koh, A., Tao, S., Ang, S.T., See, K., Kathiresan, P., Orbán, L., Wohland, T., and Winkler, C. (2021). A Neurexin2aa deficiency results in axon pathfinding defects and social impairment in zebrafish. Human Molecular Genetics 29, 3765-3780.
-
Schartl, M., Kneitz, S., Ormanns, J., Schmidt, C., Anderson, J., Amores, A., Catchen, J., Wilson, C., Geiger, D., Du, K., Garcia, M., Sundaram, S., Winkler, C., Hedrich, R., Warren, W., Walter, R., Meyer, A., Postlethwait, J.H. (2021). The developmental and genetic architecture of the sexually selected male ornament of swordtails. Current Biology 31, 911-922.
-
Witten, P.E., Huysseune, A., Maisey, J.G., Winkler, C., Gong, Z. (2021). A Boost for Fish Skeletal Research. J Fish Biol 98, 903-905.
-
Imangali N, Phan QT, Mahady G, Winkler C. (2021). The dietary anthocyanin delphinidin prevents bone resorption by inhibiting Rankl-induced differentiation of osteoclasts in a medaka (Oryzias latipes) model of osteoporosis. J Fish Biol 98, 1018-1030.
-
Pham, C.V., Pham, T.T., Lai, T.T., Trinh, D.C., Nguyen, H.V.M., Ha, T.T.M., Phuong, T.T., Tran, L.D., Winkler, C., To, T.T. (2021). Icariin reduces bone loss in a Rankl-induced transgenic medaka (Oryzias latipes) model for osteoporosis. J Fish Biol 98, 1039-1048.
-
Phan, Q.T., Tan, W.H., Liu, R.R., Sundaram, S., Buettner, A., Kneitz, S., Cheong, B., Vyas, H., Mathavan, S., Schartl, M., Winkler, C. (2020). Cxcl9l and Cxcr3.2 regulate recruitment of osteoclast progenitors to bone matrix in a medaka osteoporosis model. Proc Natl Acad Sci USA 117, 19286-19286.
-
Phan, Q.T., Liu, R., Tan, W.H., Imangali, I, Cheong, B., Winkler, C. (2020). Macrophages switch from an immune to an osteo-modulatory profile upon Rankl induction in a medaka (Oryzias latipes) osteoporosis model. JBMRPlus 4(11):e10409.
-
Mo, J., Au, D.W.T, Wan, M.T., Shi, J., Zhang, G., Winkler, C., Kong, R.Y.C, Seemann, F. (2020). Multigenerational impacts of benzo[a]pyrene on bone modeling and remodeling in medaka (Oryzias latipes). Env Sci Tech 54, 12271-12284.
-
Lleras-Forero, L., Winkler, C. and Schulte-Merker, S. (2020). Zebrafish and medaka as models for biomedical research of bone diseases. Developmental Biology 457, 191-205.
