
Zhao Ziqing Winston (CBIS)
Principal Investigator
Assistant Professor (NUS Presidential Young Professorship), Department of Chemistry; Principal Investigator, Centre for Bioimaging Sciences
Research Areas: Single-molecule/single-cell imaging; super-resolution nanoscopy; chromatin organization and dynamics; gene expression regulation; cell nuclear architecture; biomolecular phase separation; cancer and aging-associated diseases
Research
The research in the Zhao group focuses on the development and application of advanced imaging-based approaches to quantitatively probe the biophysics of chromatin dynamics at the single-cell level. In particular, we are interested in understanding how molecular processes related to chromatin structure, accessibility, and expression are organized and regulated in space and time. By integrating approaches from optical microscopy and spectroscopy (e.g. super-resolution imaging, fluorescence correlation spectroscopy and single-molecule tracking), genome and protein engineering, omic techniques and computational analysis, our work aims to illuminate the physico-chemical driving forces that underpin the spatio-temporal heterogeneities in chromatin dynamics, as well as the physiological implications of their misregulation in human diseases (particularly cancer and aging-associated diseases).
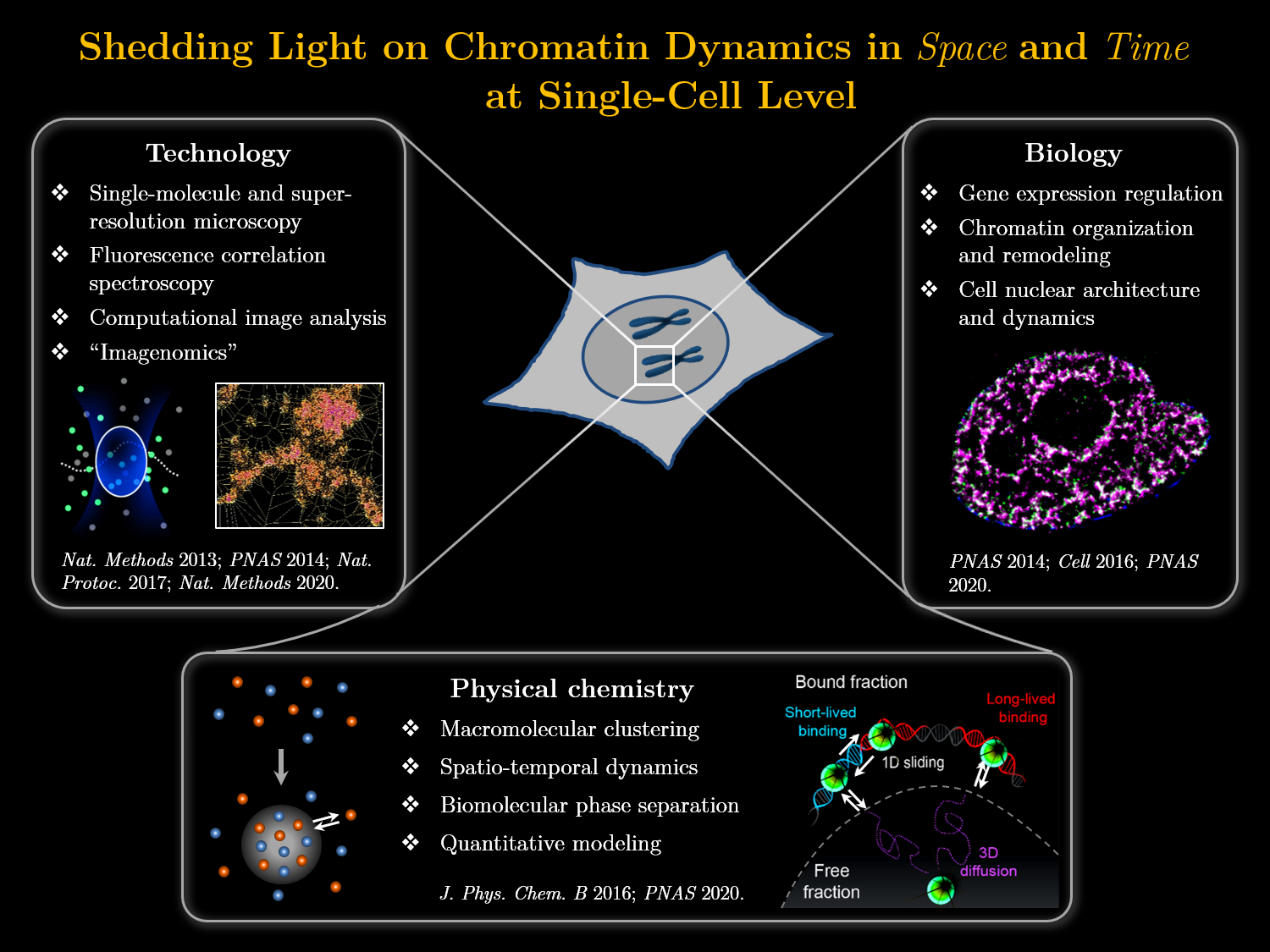
Our research interests intersect as well as span imaging technology, molecular biology, and physical chemistry. Over the years, we have developed a series of novel imaging techniques that overcome the critical challenges posed by the high molecular density and fluorescence background in the mammalian cell nucleus. As such, they have enabled single-molecule and super-resolution imaging of key cell nuclear structures, as well as the monitoring of highly transient DNA–protein interactions with superior sensitivity in complex systems such as live embryos. Using these techniques, we mapped the spatial organizations of gene transcription and DNA replication at sub-diffraction-limit resolution, and revealed critical temporal changes in the binding dynamics of transcription factors that predict cell fates over the course of early embryonic development.
Continuing with the focus on molecular processes that modulate the human genome, current work in the group is directed towards applying these approaches to dissect the spatio-temporal organization and dynamics of chromatin remodeling and nuclear membrane-chromatin interactions in single cells, as well as developing novel tools and frameworks to elucidate the mechanistic paradigms (e.g. biomolecular phase separation) that regulate such dynamics in vivo.
Selected Publications
Goh, J. J. L., Chou, N., Seow, W. Y., Ha, N., Cheng, C. P. P., Chang, Y.-C., Zhao, Z. W., Chen, K. H. Highly specific multiplexed RNA imaging in tissues with split-FISH. Nature Methods 17:689–693 (2020).
Su, Q. P.*,§ , Zhao, Z. W.*,§, Meng, L., Ding, M., Zhang, W., Li, Y., Liu, M., Li, R., Gao, Y.-Q., Xie, X. S.§, Sun, Y§. Superresolution imaging reveals spatiotemporal propagation of human replication foci mediated by CTCF-organized chromatin structures. Proc. Natl. Acad. Sci. U.S.A. 117:15036–15046 (2020). (*: equal contribution; §: co-corresponding author)
White, M. D.*, Zhao, Z. W.*, Plachta, N. In vivo imaging of single mammalian cells in development and disease. Trends Mol. Med. 24:278–293 (2018) (cover article). (*: equal contribution)
Zhao, Z. W.*, White, M. D.*, Alverez, Y. D.*, Zenker, J.*, Bissiere, S., Plachta, N. Quantifying transcription factor–DNA binding in single cells in vivo with photoactivatable fluorescence correlation spectroscopy. Nature Protoc. 12:1458–1471 (2017) (*: equal contribution).
White, M. D., Angiolini, J. F., Alverez, Y. D., Kaur, G., Zhao, Z. W., Mocskos, E., Bruno, L., Bissiere, S., Levi, V., Plachta, N. Long-lived binding of Sox2 to DNA predicts cell fate in the four-cell mouse embryo. Cell 165:75–87 (2016) (cover article).
Zhao, Z. W.,Xie, X. S., Ge, H. Nonequilibrium relaxation of conformational dynamics facilitates catalytic reaction in an elastic network model of T7 DNA polymerase. J. Phys. Chem. B 120:2869–2877 (2016).
Zhao, Z. W.*, Roy, R.*, Gebhardt, J. C. M.*, Suter, D. M.*, Chapman, A. R., Xie, X. S. Spatial organization of RNA polymerase II inside a mammalian cell nucleus revealed by reflected light-sheet superresolution microscopy. Proc. Natl. Acad. Sci. U.S.A. 111:681–686 (2014) (*: equal contribution).
Zhao, Z. W., Gebhardt, J. C. M., Suter, D. M., Xie, X. S. Reply to “Convergence of chromatin binding estimates in live cells”. Nature Methods 10:692 (2013).
Gebhardt, J. C. M., Suter, D. M., Roy, R., Zhao, Z. W., Chapman, A. R., Basu, S., Maniatis, T., Xie, X. S. Single-molecule imaging of transcription factor binding to DNA in live mammalian cells. Nature Methods 10:421–426 (2013).
