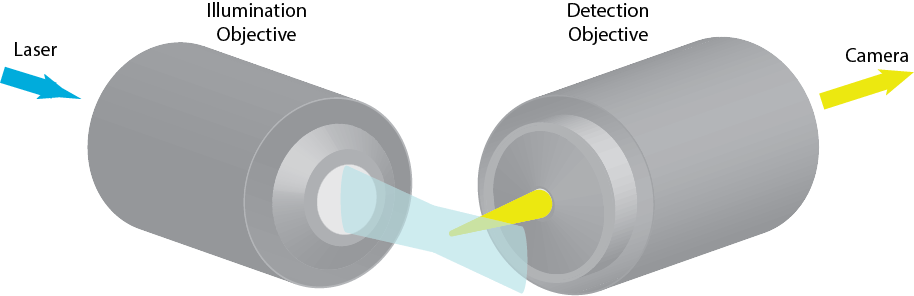
Imaging FCS excites fluorescence in a cross-section of a sample. The cross-section is then imaged onto a camera or array detector, which records the data at high speed. If the cross-section is sufficiently thin (<1 µm), the camera sufficently fast (~1 ms per frame or faster) and the pixels on the order of the diffraction limit, the observation volume of each pixel is sufficiently small and the time resolution sufficiently fast to detetc single molecule fluctuations and calculate ACFs for each pixel.
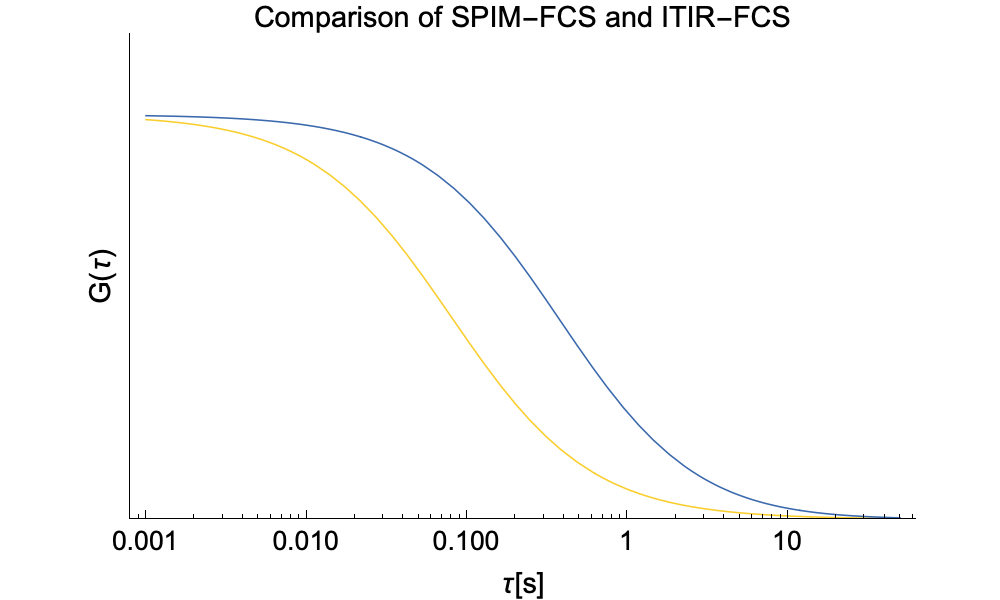
The ACFs in SPIM-FCS exhibit the same general characteristics as the ones in ITIR-FCS. The main difference is that in the case of SPIM-FCS we need to take into account movement along the optical axis of the detection objective and thus study 3D diffusion.
In a first approximation, the effective observation volume is given by:
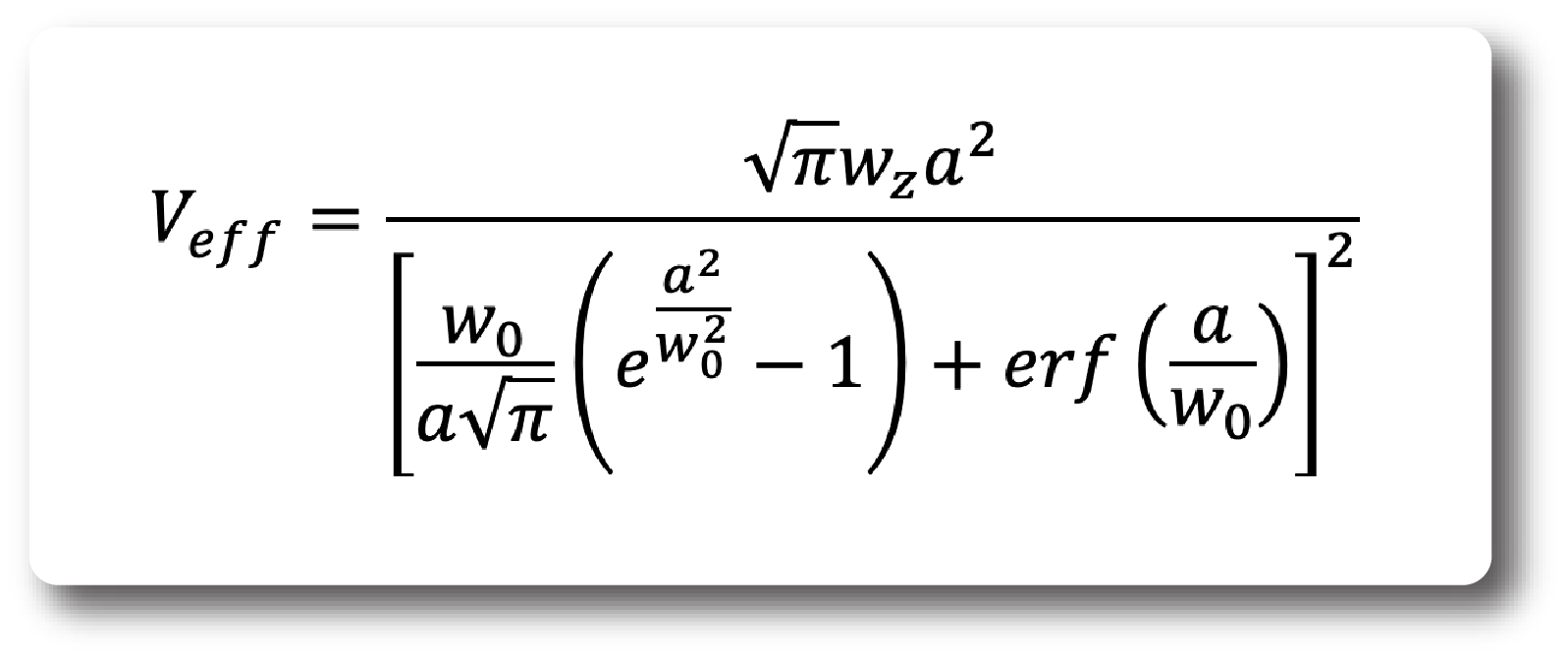
This is only an approximation as it disregards the fact that the actual intensity in lateral direction (perpendicualr to the optical axis of the detection objective) is constant and thus in reality leads to cross-talk, that reduces the amplitude and increases the width of the ACF. This can lead to underestimations of the diffusion coefficient on the order of 30% in our setups. However, this is especially visible in CCFs where the cross-talk contribution at short times is larger than would be expected from the equation above.
The approximate ACF for SPIM-FCS is given by:
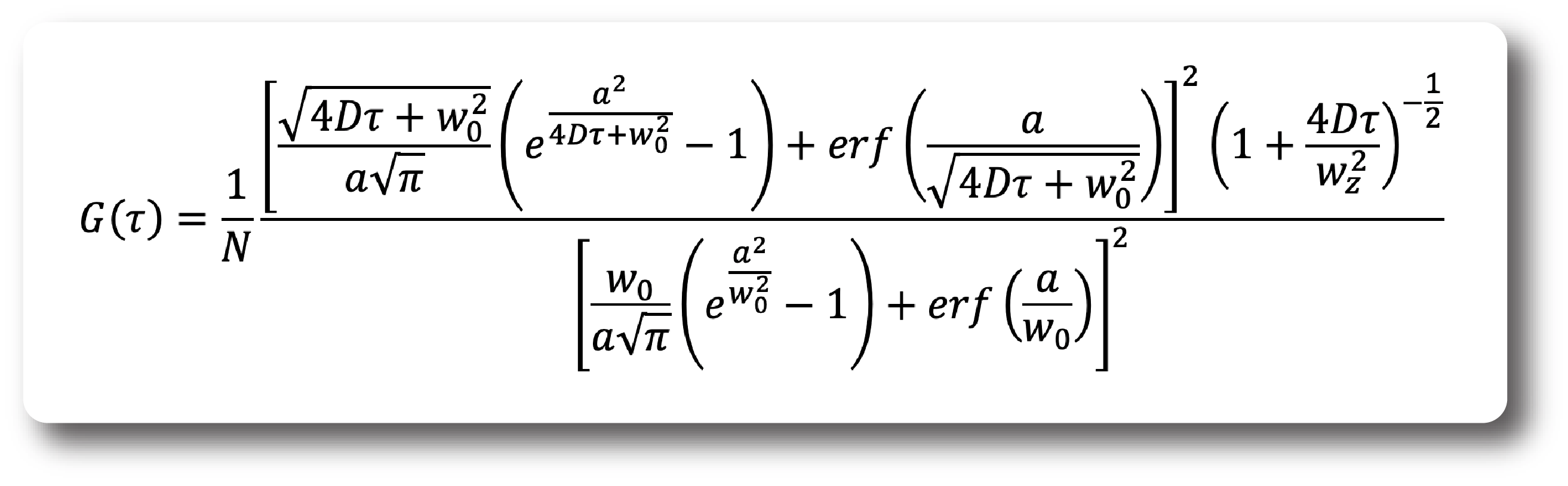
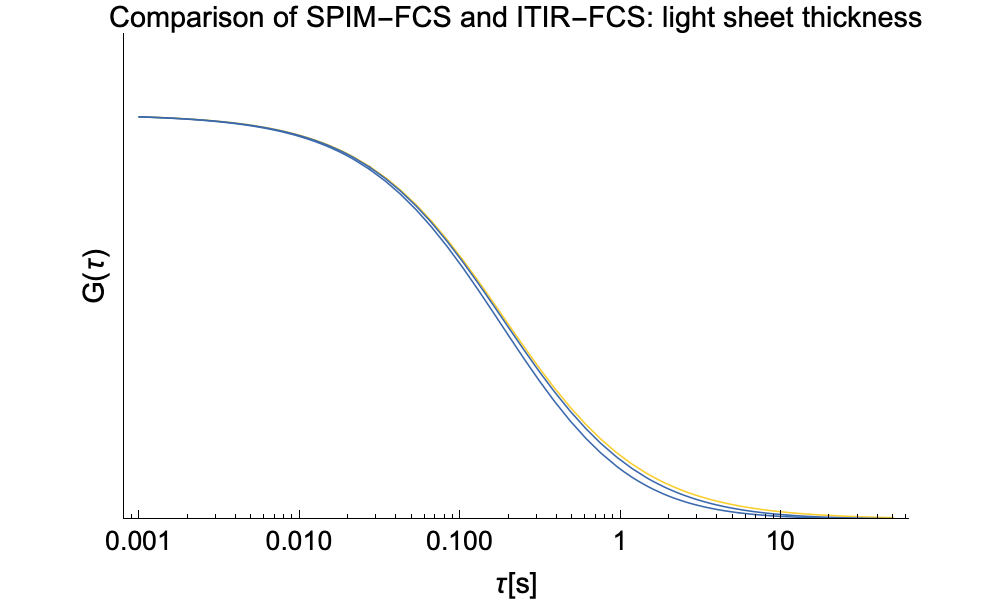
As we found no analytic solution for the cross-talk problem we resorted to numerical integration. Below we provide a comparison of SPIM-FCS functions with or without cross-talk.