We have purified new family of potassium channel
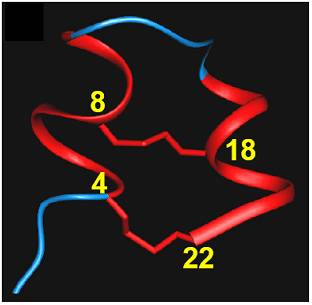
blockers from scorpi
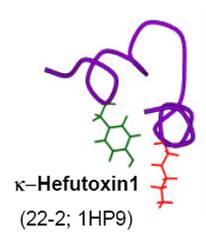
on venoms. They are structurally unrelated any known proteins. Based on their 3D structure, we studied the presence of lysine and phenylalanine dyad and predicted its ability to block the potassium channels. We have shown that these short peptides block Kv1.2 and Kv1.3 channels. We also described the importance of the dyad for its function.

