Using zebrafish models to study the disease mechanism of Spinal Muscular Atrophy (SMA)
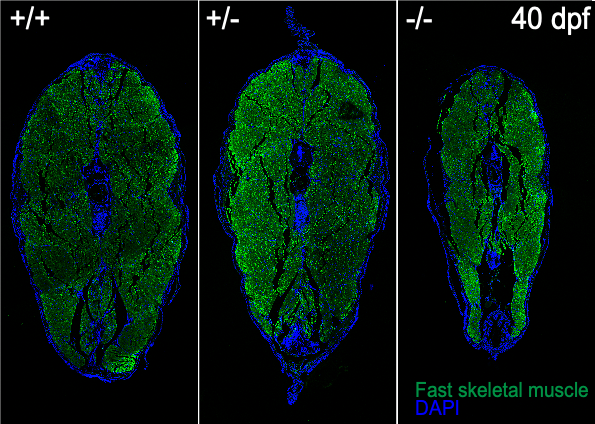
Image: Muscle atrophy in 40 days old mutant.
SMA is a neurodegenerative disease that represents the leading genetic cause for infant mortality. SMA is caused by reduced levels of a ubiquitous protein, SMN, and severely affects the neuromuscular system with a characteristic motor neuron degeneration. We established a zebrafish model of SMA that due to its optical clarity allows to capture various developmental processes in real time through non-invasive live imaging techniques.
Using CRISPR-Cas9, we generated a mutant line zfsmnA6T that recapitulates aspects of human intermediate Type II SMA. This mutant presents a progressive loss of SMN protein (to ~20%) at late juvenile and early adult stages, which consequently leads to defects in the maintenance of pre-synapses commencing at late juvenile stages.
Using this unique model, we aim to identify essential SMN downstream target genes that control the maintenance of pre-synaptic nerve endings, Schwann cell dynamics and motor neuron development. Currently, we examine the formation and maintenance of neuromuscular junctions as well as axonal transport in SMN-deficient motor neurons in the zfsmnA6T mutants.
Understanding the mechanisms of axonal transport in developing neurons
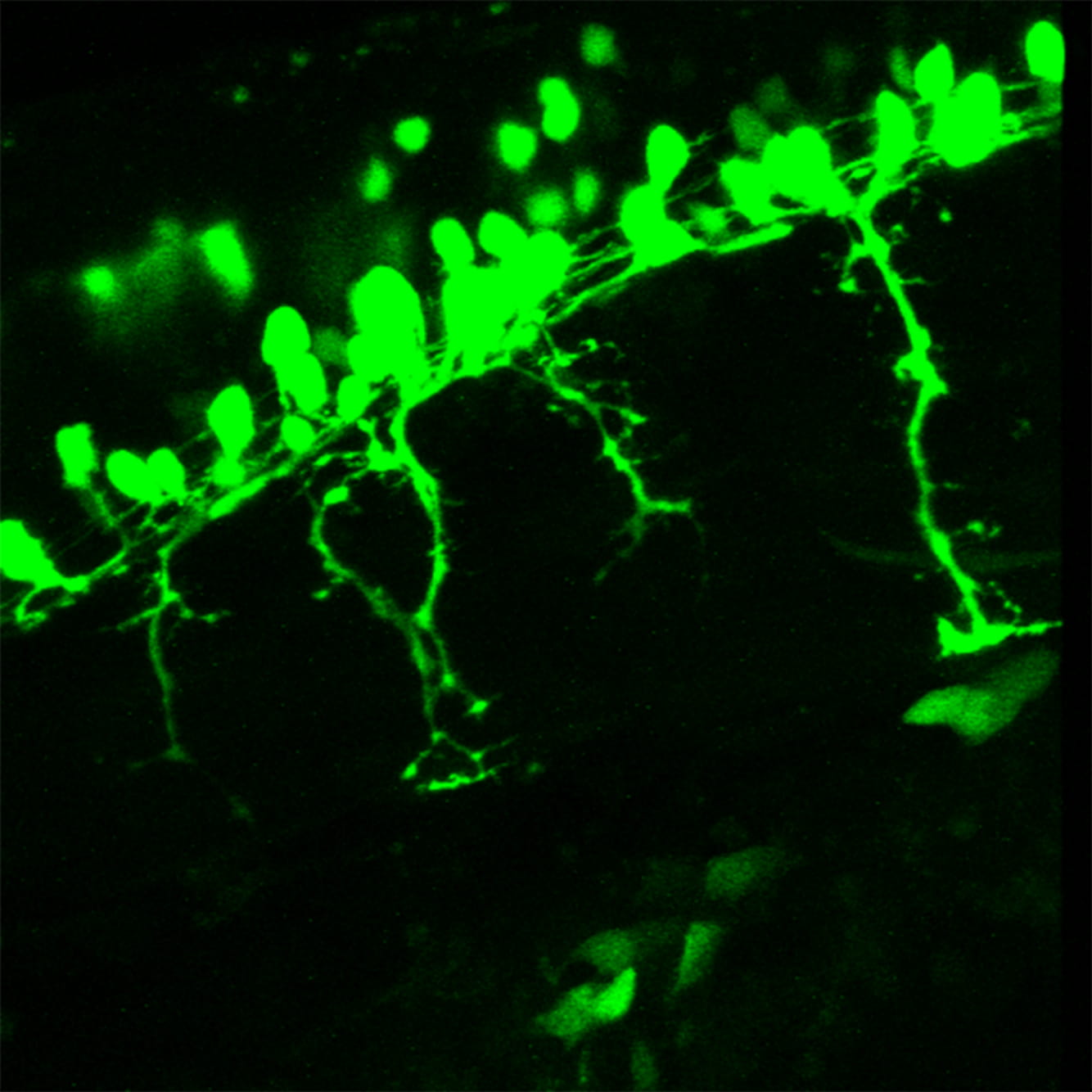
Image: Flourescent cell bodies and branched axons.
In axonal transport, motor proteins actively deliver cargoes such as organelles, vesicles and cytoskeletal proteins along microtubules, from one end of the axon to the other. This process is essential for neuronal development and survival. Disruptions in cargo transport have been reported in many neurodegenerative disorders and we are interested to understand the molecular aspects of how axonal transport contributes to maintaining neuron integrity.
We established several genetic and molecular tools in the zebrafish model that enable us to visualise and understand how specific adaptor proteins are important for axonal transport.
In vivo characterization of zebrafish Mdk/Ptn-Ptprz1 signalling in embryogenesis
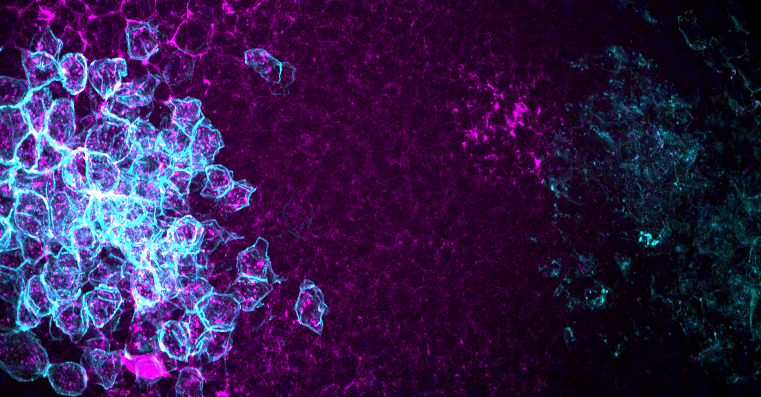
Image: Modelling of Mdk diffusion in vivo. Mdk (coloured in magenta) diffuses from the source cells (coloured in cyan) on the left side to the receptor expressing cells (also coloured in cyan) on the right.
Midkine (MDK) and pleiotrophin (PTN) are secreted heparin-binding growth factors. They are proposed to function in several biological processes, such as embryogenesis and cancer metastasis, through binding to receptor protein tyrosine phosphatase zeta 1 (PTPRZ1). The exact role of this ligand-receptor interaction, however, remains elusive.Due to a whole-genome duplication, zebrafish possesses two mdk genes (mdka and mdkb), one ptn, and two ptprz1 genes (ptprz1a and ptprz1b), which implies potential sub- and neo-functionalizations with different ligand-receptor combinations.
In this project, we utilized the CRISPR-Cas9 system to generate various mdk/ptn knockout and knockin zebrafish lines to characterize their loss-of-function phenotypes and to visualize endogenous Mdk/Ptn dynamics in vivo. These models allow us to gain a better understanding of the dynamics of Mdk/Ptn diffusion and their binding to Ptprz1 in vivo with the help of cutting-edge live imaging and biochemical techniques. Our findings will provide new insight into the functions of Mdk/Ptn-Ptprz1 signalling during zebrafish embryogenesis.
In vivo imaging of osteoblast-osteoclast interaction in a medaka model for osteoporosis
Video: Dynamic interation of bone and immune cells
We are interested in the cellular mechanisms that control bone homeostasis. Bone cells of fish, such as the medaka, have morphological and physiological features that are highly similar to those of human bone cells. Also, the genetic networks that regulate formation of bone-forming osteoblasts and bone-resorbing osteoclasts are highly conserved.
We have established several transgenic medaka lines that express fluorescent reporters in various bone cells at distinct stages of differentiation. We also generated a medaka osteoporosis model, in which the osteoclast-inducing factor RANKL is expressed under control of a heatshock promoter. Upon heatshock, RANKL induces the formation of ectopic osteoclasts. This results in excessive degradation of bone matrix in a manner very similar to the situation in human osteoporosis patients.
This unique in vivo model allows visualization of osteoblast/osteoclast interaction in an intact living animal model during bone degradation as well as regeneration. Using this model, we have identified several novel factors that control the recruitment of progenitor cells to bone matrix and maintain bone homeostasis.
A medaka model for Schmid Metaphyseal Chondrodysplasia (SMCD)
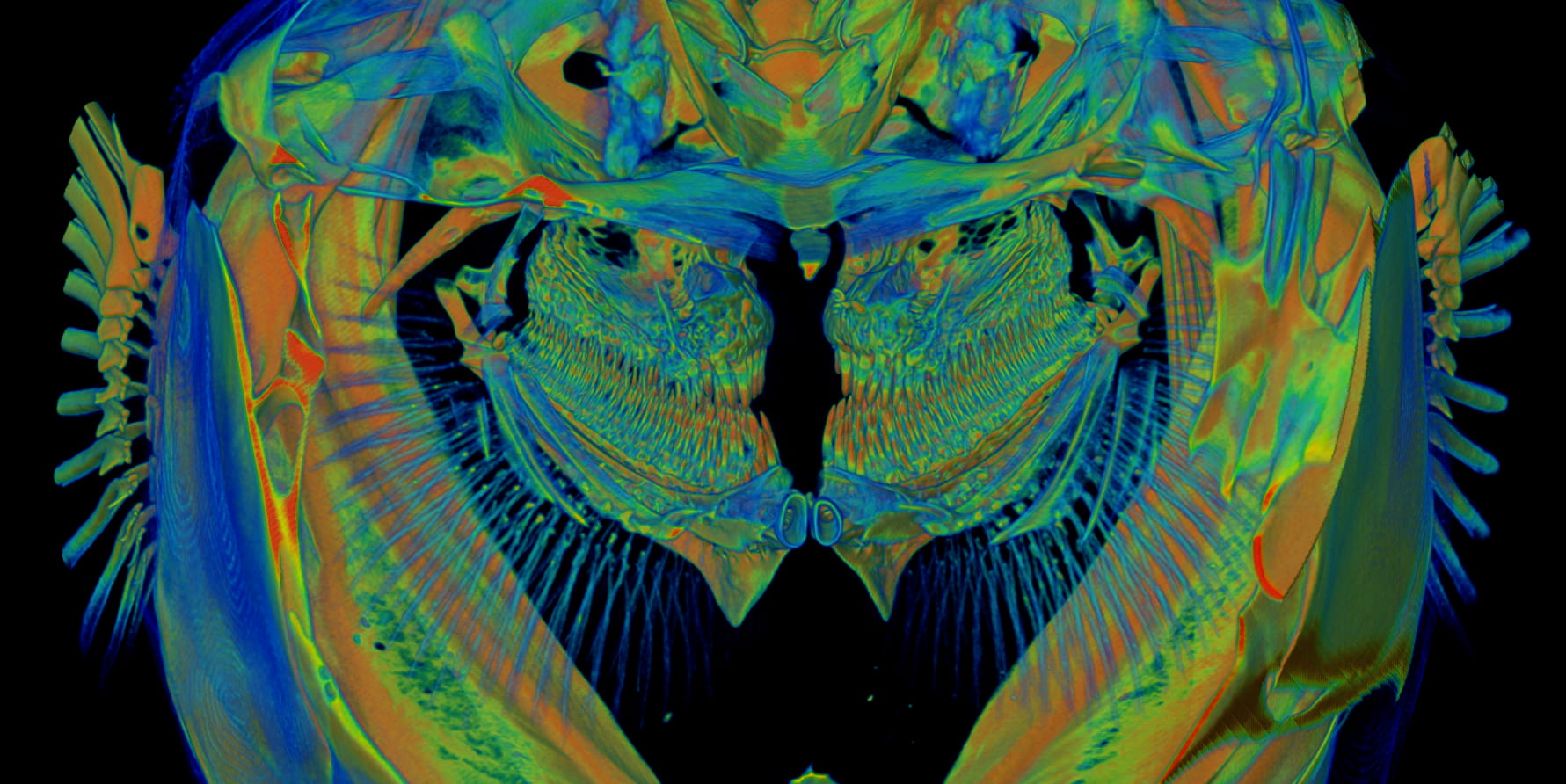
Image: MicroCT image of medaka gills.
SMCD is a skeletal disease associated with bone growth plate abnormalities, hip deformities and dwarfism. At present, the pathomechanism of SMCD remains unclear and there is no cure for this disease. To elucidate the molecular mechanisms underlying SMCD, we generated a medaka mutant that phenocopies SMCD.
Using high-resolution imaging techniques such as live confocal microscopy, X-ray tomographic microscopy and electron microscopy, we found that cell polarity is impaired in the SMCD medaka mutant. Further investigation into the cause and consequences of disrupted cell polarity in the medaka mutant may shed light on the pathogenesis of SMCD and facilitate development of future SMCD therapies.