|
|
Origin and Evolution of Prothrombin
Activators
|
Snake venom prothrombin
activators can be classified in to four groups based on
their structure, cofactor requirements and the products
formed. Groups A and B are metalloproteases, whereas
groups C and D are serine proteases. The latter two
groups have been found only in Australian elapids. We
showed that group D prothrombin activators are
structural and functional homologs of blood coagulation
factor Xa. Subsequently, we also showed that group C
prothrombin activators are similar to mammalian factor
Xa-factor Va complex. These prothrombin activators
induce microclots leading to disseminated intravascular
coagulopathy, cyanosis and death. Thus they are used as
toxins. As snakes also have a blood coagulation system,
they possess factor X and factor V which would play
critical role in their hemostatic mechanism in their
plasma. Our studies have linked origin and evolution of
these two parallel prothrombin activator systems which
play two distinct physiological roles in Australian
elapids.
-
We have shown that the two
parallel prothrombin activator systems are
structurally similar with subtle differences. For
example, the activation p
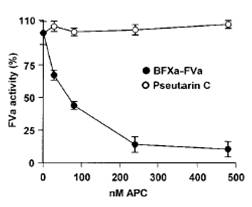 eptide of the venom protein
is much shorter than that of the liver factor X.
Further, there is an insertion of 11-residue segment
in the heavy chain. The implications of these
changes are unclear. Interestingly, sites for
proteolytic inactivation by activated protein C (APC)
are modified in the venom factor V but not in plasma
factor V protein making the venom prothrombin
activator resistant to inactivation by APC. This
provides a distinct advantage for their use as
toxins. Thus these venom prothrombin activators
(group C and D) have evolved by gene duplication of
coagulation factors and recruited to be expressed in
the venom glands. eptide of the venom protein
is much shorter than that of the liver factor X.
Further, there is an insertion of 11-residue segment
in the heavy chain. The implications of these
changes are unclear. Interestingly, sites for
proteolytic inactivation by activated protein C (APC)
are modified in the venom factor V but not in plasma
factor V protein making the venom prothrombin
activator resistant to inactivation by APC. This
provides a distinct advantage for their use as
toxins. Thus these venom prothrombin activators
(group C and D) have evolved by gene duplication of
coagulation factors and recruited to be expressed in
the venom glands.
-
During our sequencing studies
of factor X gene from Ps
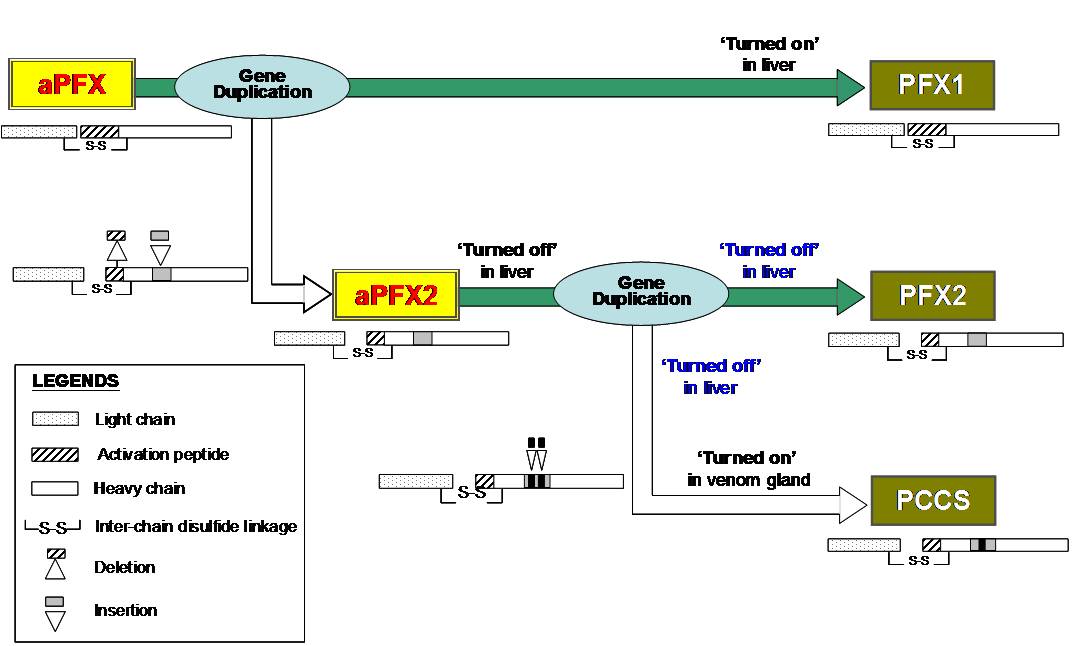 eudonaja textilis
liver, we found two sequences, PFX1 and PFX2. PFX2
has several characteristics similar to venom gene
while PFX1 is similar to the factor X gene of other
snake factor X. The activation peptide segment in
PFX2 is shorter in size, similar to venom protein.
There is a smaller insert in the heavy chain. Thus
PFX2 appears as an intermediate in the evolution of
the factor Xa-like enzymatic subunit of prothrombin
activators. eudonaja textilis
liver, we found two sequences, PFX1 and PFX2. PFX2
has several characteristics similar to venom gene
while PFX1 is similar to the factor X gene of other
snake factor X. The activation peptide segment in
PFX2 is shorter in size, similar to venom protein.
There is a smaller insert in the heavy chain. Thus
PFX2 appears as an intermediate in the evolution of
the factor Xa-like enzymatic subunit of prothrombin
activators.
- Using real-time PCR we showed that the above proteins are expressed with high tissue-specificity in the liver and venom glan
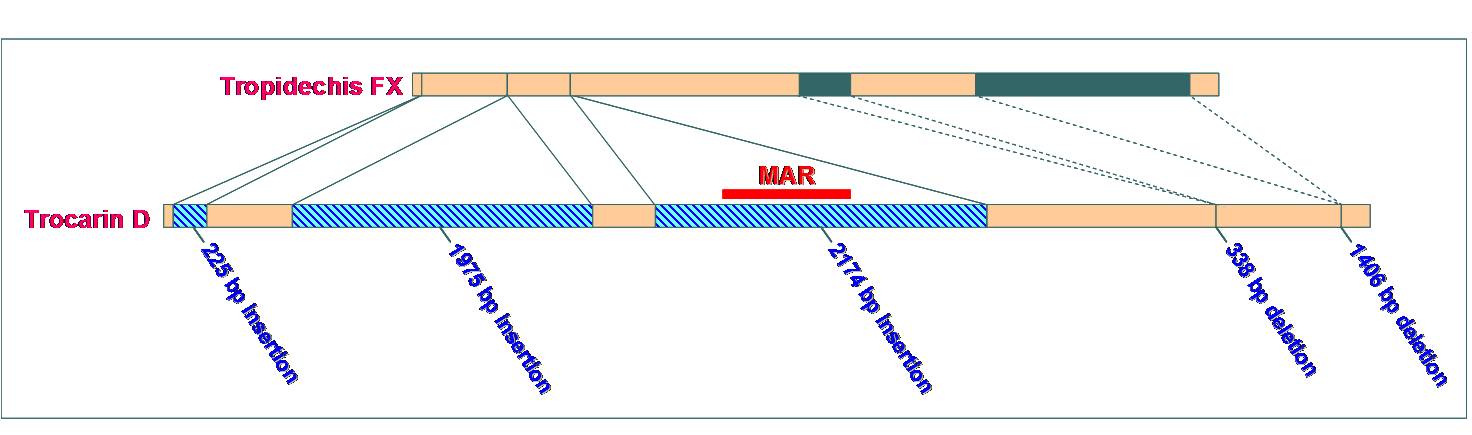 ds. To understand their expression and regulation, we completed the gene sequence of trocarin D, catalytic subunit of pseutarin C and factor Xs from Tropidechis carinatus and Pseudonaja textilis snakes. The venom genes have a insert containin ds. To understand their expression and regulation, we completed the gene sequence of trocarin D, catalytic subunit of pseutarin C and factor Xs from Tropidechis carinatus and Pseudonaja textilis snakes. The venom genes have a insert containin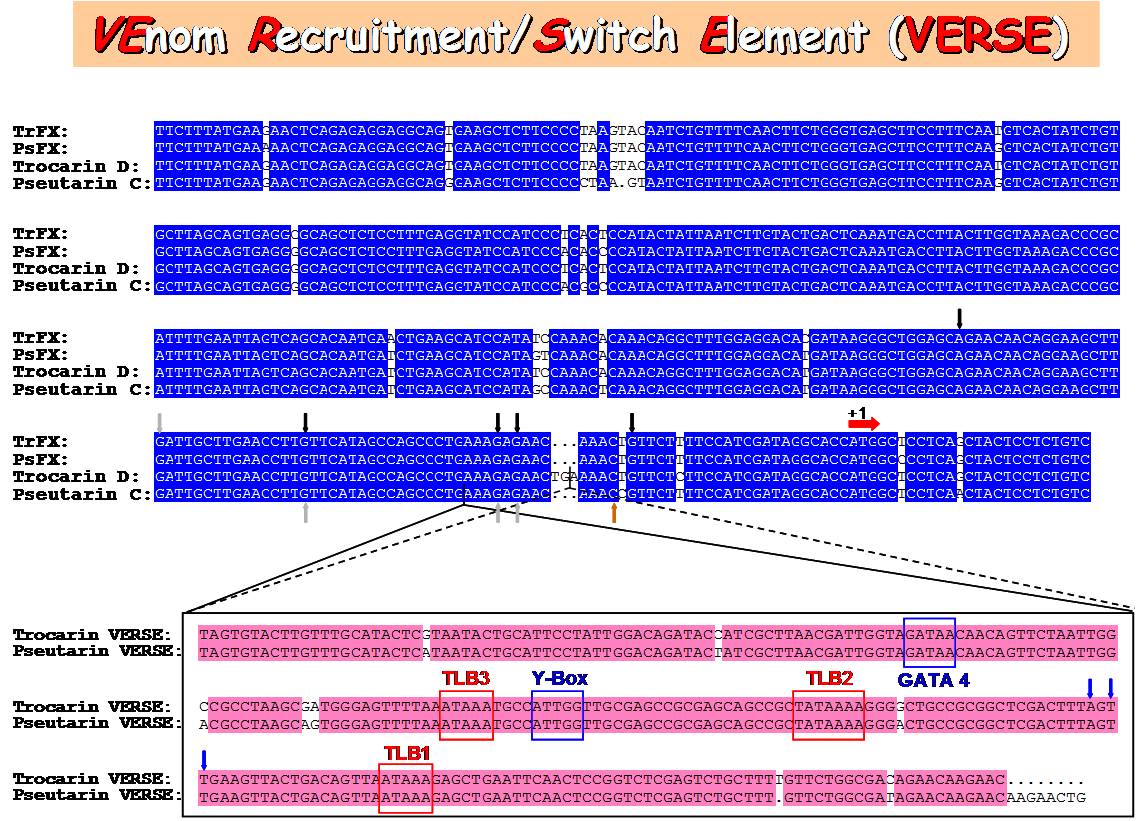 g three TATA-like boxes, a GATA box and Y-box in the 5’-upstream region. We identified this segment as VERSE (Venom Recruitment/Switch Element).In addition, the venom genes have three insertions and two deletions in their first introns compared to liver factor X gene. We are currently attempting understand the role of these differences in tissue-specific expression and regulation. g three TATA-like boxes, a GATA box and Y-box in the 5’-upstream region. We identified this segment as VERSE (Venom Recruitment/Switch Element).In addition, the venom genes have three insertions and two deletions in their first introns compared to liver factor X gene. We are currently attempting understand the role of these differences in tissue-specific expression and regulation.
|
Key
Publications
|
|

