|
Snake venom PLA2 enzymes are esterolytic enzymes which hydrolyze glycerophospholipids at the sn-2 position of the glycerol backbone releasing lysophospholipids and fatty acids. So far, several hundred snake venom enzymes have been purified and characterized. They share similarity in structure and catalytic function with mammalian enzymes. However, in contrast to mammalian enzymes, many are toxic and induce a wide spectrum of pharmacological effects.Our work has significantly contributed to the structure-function relationships and mechanism of PLA2 enzymes.
-
We described a hypothetical model to explain various pharmacological effe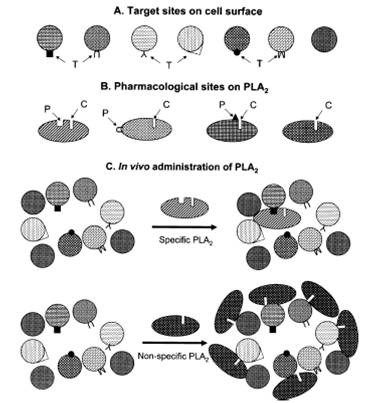 cts of snake venom PLA2 enzymes. Various pharmacological effects are defined by protein-protein interaction between PLA2 and protein acceptor/receptor on the target cell surface, unlike previous hypotheses, which gave importance to protein-phospholipid interactions. Others and we have provided experimental evidence for the model in recent years. The paper describing the model has provided impetus in the field and it changed the direction in the field cts of snake venom PLA2 enzymes. Various pharmacological effects are defined by protein-protein interaction between PLA2 and protein acceptor/receptor on the target cell surface, unlike previous hypotheses, which gave importance to protein-phospholipid interactions. Others and we have provided experimental evidence for the model in recent years. The paper describing the model has provided impetus in the field and it changed the direction in the field [ Phospholipase A2 (PLA2) enzymes (Target model)].
-
We developed theoretical methods to solve structure-function relationships of snake venom PLA 2 enzymes. We have identified neurotoxic, myotoxic, anticoagulant and antiplatelet sites. The paper on antiplatelet site is yet to be published. Anticoagulant and antiplatelet peptides are covered under two US patents, whereas we have applied for the third patent for the antiplatelet peptide. For details, see Phospholipase A2 (PLA2) enzymes .
-
We determined the mechanism of anticoagulant effects of a snake venom PLA 2 e  nzyme. The strongly anticoagulant enzyme specifically inhibits the prothrombinase complex, whereas weakly anticoagulant enzymes do not. This inhibition is independent of binding to or hydrolysis of phospholipids. Strongly anticoagulant PLA 2 binds to blood coagulation factor Xa and interferes in its interaction with coagulation factor Va, thus impeding the formation of the prothrombinase complex. This is the first anticoagulant that interferes in the formation of a coagulation complex. For details, see Anticoagulant phospholipase A2 enzymes.
-
By comparing the structures of 127 snake venom PLA 2 enzymes, we showed tha  t mutational hot spots occur on the surface of this protein molecule. Natural substitutions occur about 2.6-3.5 times greater in fully exposed residues than in the buried residues. The surface substitutions and the accelerated evolution of exons play a significant role in the evolution of new PLA 2 isoenzymes by altering the target specificity.
-
Descri  bed the edema inducing effects of PLA 2 enzymes. They are some of the first papers describing the role of PLA 2 enzymes in edema and inflammatory diseases. We also described the neutralization of edema by aristolochic acid, a plant alkaloid.
|

