ITIR-FCS
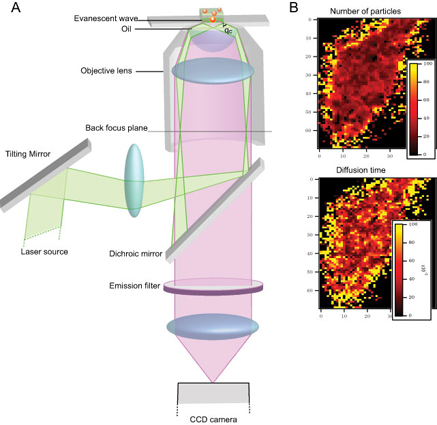
Conventional FCS instruments use point detectors thus making multiplexing of measurements difficult. Electron multiplying charge coupled device (EMCCD) cameras possess the necessary speed and detection efficiency needed for FCS and could allow the measurement of thousands of FCS curves simultaneously. However, for the recording of an FCS curve the observation volume must be small to allow single molecule sensitivity. In conventional FCS this is provided by the laser focus and the confocal pinhole. In EMCCD camera based FCS the limitation of the observation volume is achieved by the finite extent of the pixels (or group of pixels) on the CCD chip and a laser illumination that provides intrinsic z-sectioning. The illumination in our instruments is achieved by the exponentially decaying evanescent wave generated due to Total Internal Reflection (TIR). We call this method, Imaging Total Internal Reflection-Fluorescence Correlation Spectroscopy (ITIR- FCS), because this method allows one to obtain quantitative images of FCS parameters.
This approach allows us to measure regions up to 100 µm2which are sufficiently large to cover an entire cell. ITIR-FCS has been applied to study diffusion and flow phenomena in supported lipid bilayers and cell membranes. This method also allows computing the cross-correlation (ITIR-FCCS) between any two pixel areas on the EMCCD chip which provides us with vectorial information. By computing cross-correlation between neighboring pixels, dynamic changes in heterogeneity on cell-membrane can also be observed.
We have two ITIR-FCS systems in our lab.
TIRFM 1
The first objective-type total internal reflection fluorescence microscope (TIRFM) is built using an inverted epi-fluorescence microscope (IX-71, Olympus) and two high NA oil-immersion objective (100x/1.45 and 100x/1.5) (Olympus, Singapore). Imaging and spectroscopy are carried out using the electron multiplying charge-coupled device (EMCCD) camera (Andor iXON 860) or Andor sCMOS Sona-11 or Hamamatsu sCMOS Orca Flash 4.0 mounted on the side-port of the microscope. The camera and microscope are controlled by the software Andor Solis. The spatial resolution of the images is 240 nm and the temporal resolution achieved is 0.56 ms for a region of interest of 21*21 pixels. This system is fitted with 488, 532 and 638 nm lasers.

The features of the CCD are tabulated in the table below.
| Number of pixels | 128x128 |
| Image capture region size | 3.1x3.1 mm2 |
| Pixel size |
24x24 µm |
|
Spatial resolution |
240 nm |
|
Temporal resolution |
0.56 ms (21x21 pixels) |
|
Magnification |
100x |
TIRFM 2
The second objective-type TIRFM is based on an Olympus inverted epi-fluorescence microscope (IX83) with a motorized TIRF illumination combiner (cell^TIRF/IX3-MITICO, Olympus) allowing simultaneous illumination with 4 laser lines (405, 491, 514 and 561 nm). The TIRF incidence angle can be adjusted independently for each line allowing setting the same penetration depth of the evanescent field for all excitation wavelengths. The system is equipped with an IX3-ZDC (Olympus) Z drift compensator preventing defocusing during long image stack acquisitions. Three cameras (2EMCCD and a scientific CMOS) and two image splitters (TwinCam and OptoSplit II, Cairn research) are available at the microscope. The image splitters can be used to split the image according to spectral region or polarization of the fluorescence either to two different cameras or on two halves of the chip of a single camera. Furthermore, the microscope is equipped with an on-stage incubator for controlling the temperature and CO2 concentration in the sample chamber during live cell imaging.

Excitation sources:
Olympus Cell* lasers: 405 nm (100 mW), 491 nm (100 mW), 514 nm
(50 mW) and 561 nm (100 mW).
Objectives:
Besides standard dry objectives (10x, 20x, 40x) there are 2 Olympus oil-immersion objectives for
TIRFM: UApoN 100x/1.49 and ApoN 60x/1.49
The features of the cameras are tabulated in the table below
|
Camera |
Hamamatsu ORCA-Flash4.0 V2 sCMOS |
Photometrics Evolve 512 EMCCD |
Andor iXon3 X-9388 EMCCD |
Andor Sona-11 sCMOS |
|
Number of pixels |
2048x2048 |
512x512 |
128x128 |
2048x2048 |
|
Pixel dimension |
6.5 μm |
16 μm |
24 μm |
11 μm |
|
Temporal resolution |
39 μs (8x2048 pixels) |
1.48 ms (8x512 pixels) |
0.56 ms (20x128 pixels) |
200 MHz (70 fps 2048x2048 pixels) |
|
Acquisition software |
ImFCS-Direct Camera Readout*, Micro-Manager, Hamamatsu HSR |
ImFCS-Direct Camera Readout*, Micro-Manager |
ImFCS-Direct Camera Readout*, Andor Solis |
ImFCS-Direct Camera Readout*, Andor Solis |

